FLT3 mutations (including FLT3-ITD and FLT3-TKD), occurring in ~30% of acute myeloid leukemia (AML) patients, signal poor prognosis due to elevated relapse risk. Albeit FLT3 tyrosine kinase inhibitors (FLT3 TKI) demonstrated efficacy in treating FLT3-mutated AML in the clinic, there are issues such as high cost, side effects, and high rate of drug resistance/relapse. Hence, an affordable, efficient treatment with lower side effects is needed. Metformin (MET), a cost-effective anti-diabetic drug with minimal side effects, has demonstrated anti-tumor effects (Dowling R et al. Cancer Res. 2007). Here we aimed to determine if combining MET with FLT3 TKI, like Gilteritinib (GLT), could enhance efficacy, reduce side effects and costs in treating FLT3-mutated AML.
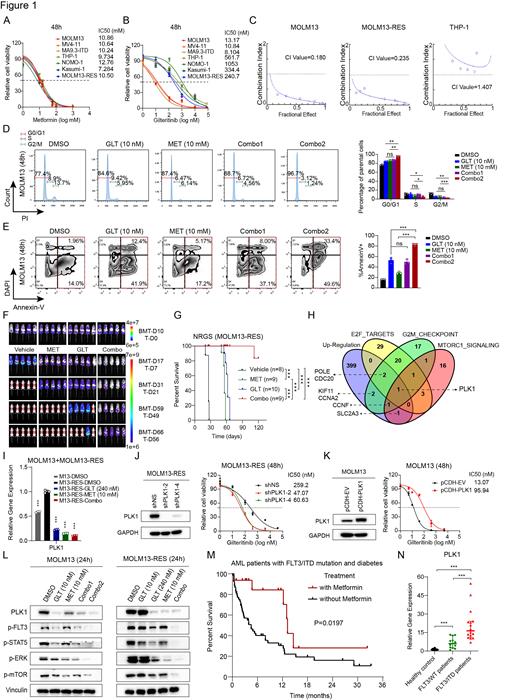
To this end, we first assessed the IC50 values of MET and GLT across various AML cell lines including those carrying FLT3/ITD (MOLM13, MV4-11, and MA9.3-ITD), FLT3 TKI resistant cells (MOLM13-RES) and those carrying FLT3 wild-type (FLT3/WT) (THP1, NOMO1 and Kasumi-1). MET's IC50 S were consistent (7.284 -12.76 mM) (Fig. 1A), while GLT's varied, with lower values in FLT3/ITD cells (10 nM) and higher in FLT3 TKI resistant (241 nM) and FLT3/WT cells (334-1,053 nM) (Fig. 1B). Using the Chou-Talalay Method, a Combination Index (CI) below 1 in FLT3/ITD and MOLM13-RES cells indicated synergy, whereas it showed antagonism (CI>1) in FLT3/WT cells (Fig. 1C). Both MET and GLT induced cell cycle arrest and apoptosis, with Combo1 (GLT 5 nM + MET 5 mM) matching GLT 10 nM effects; Combo2 (GLT 10 nM + MET 10 mM) amplified these effects (Fig. 1D and E).
We next evaluated the potential synergistic effect of MET and GLT in treating TKI-resistance AML in vivo using NRGS mice xeno-transplanted with MOLM13-RES cells. Both bioluminescence imaging and flow cytometry assays showed each treatment alone reduced leukemia burden, and their combination had a synergistic effect, reducing AML burden dramatically (Fig. 1F). While MET or GLT alone doubled overall survival compared to vehicle treatment (56-62 days vs. 26 days), ~90% of the mice treated with the Combo survived over 120 days (Fig. 1G) and their leukemia burden was still minimal, highlighting the synergistic effect and the promising therapeutic potential of this combination in treating FLT3-mutated AML (even with TKI-resistance).
To understand the underlying molecular mechanism(s), we performed RNA-seq with vehicle- or drug-treated MOLM13-RES cells, followed by pathway analysis and validation/mechanistic studies, we identified Polo-like kinase 1 (PLK1) as a key target of MET and GLT (Fig. 1H). PLK1's expression is notably suppressed by both treatments (Fig. 1I). Mimicking MET treatment, PLK1 knockdown enhanced GLT sensitivity, reducing IC50 from 259.2 nM to 47-60 nM (Fig. 1J). Conversely, PLK1 overexpression increased GLT's IC50 7-fold (13.07 nM to 95.94 nM) (Fig. 1K), largely reversing MET's effect (data not shown). PLK1, a crucial serine/threonine-protein kinase, influences multiple signaling pathways such as MAPK and mTOR pathways. Upon treating MOLM13 or MOLM13-RES cells with MET and GLT alone or combined, the PLK1 levels and phosphorylation levels of FLT3, STAT5, ERK, and mTOR were decreased, with the most significant decrease occurred upon the combined treatment (Fig. 1L). Depletion and overexpression of PLK1 decreased and increased these phosphorylation levels, respectively (data not shown). Together, our findings suggest that the synergistic therapeutic effect of MET and GLT in treating FLT3-mutated AML is likely through synergistically targeting PLK1 and its downstream pathways.
Meanwhile, we executed a retrospective clinical study of FLT3/ITD AML patients who were also diagnosed with diabetes. Metformin users showed a notably longer median OS (13.2 months) than non-MET users (4.5 months, P=0.0197) (Fig. 1M). Despite FLT3/ITD status, FLT3 TKIs were seldom used in China due to high cost. Additionally, PLK1 level was dramatically higher in FLT3/ITD AML cases than in both healthy and FLT3/WT cases (P<0.001) (Fig. 1N), implying PLK1's role in FLT3/ITD's poor prognosis.
In summary, our findings suggest a potential synergistic approach in treating FLT3-mutated AML using MET and GLT. This approach could reduce side effects and costs (crucial for developing countries) and improve outcomes. Larger clinical trials are needed to confirm these promising initial findings.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal